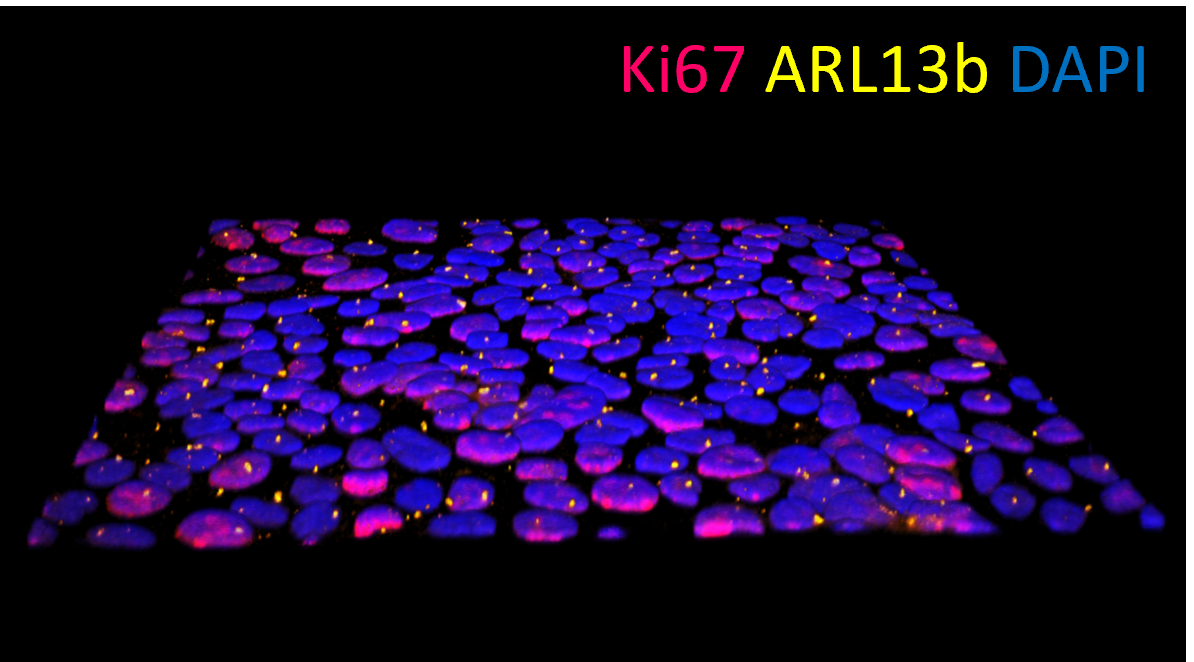
Researchers in the BIRDIE consortium have created a versatile stem cell-based model of the kidney proximal tubule. The study, published in Toxicology In Vitro, shows that the model can accurately predict the toxic effect of multiple reference compounds. Additionally, the study highlights the need for precise modeling of the dynamic flow conditions present in the kidney to provide further insight into nephrotoxicity mechanisms.
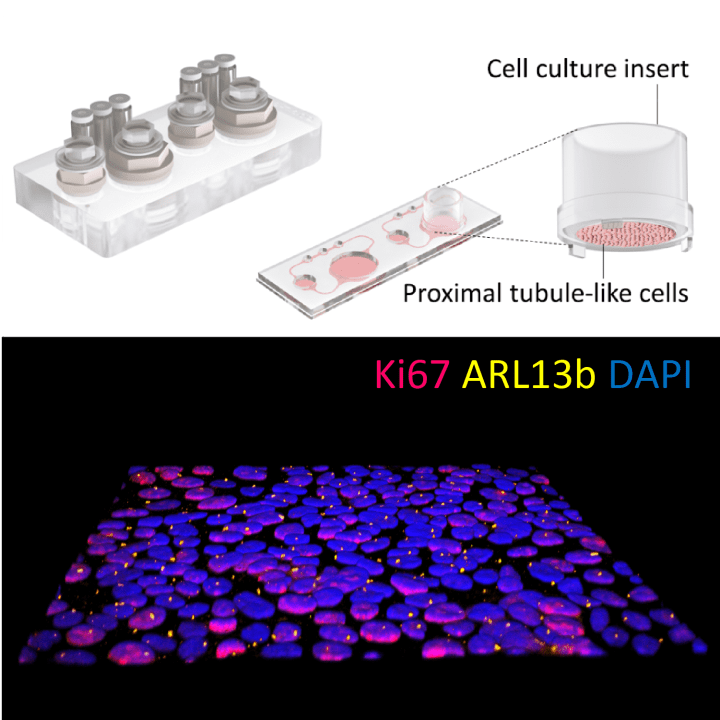
Our kidneys play an important role in the way our bodies metabolize drugs. Having research models that can detect kidney toxicity early during drug development is therefore of key importance. In a new study, researchers in the EU-funded BIRDIE consortium have created a stem cell-derived model of the kidney proximal tubule that is easy to integrate in a microphysiological system. The researchers tested the model using multiple reference compounds, demonstrating its capacity to drug-induced nephrotoxicity.
“Our results show that our kidney-on-chip model yield very robust and reproducible results and is well-suited to investigate nephrotoxicity. We evaluated the model using three different known kidney toxicants: the antibiotic polymyxin B, the immunosuppressant cyclosporin A, and the chemotherapeutic agent cisplatin” says Eva-Maria Dehne, corresponding author and senior researcher at TissUse GmbH.
A central function of the kidney is filtering blood from toxins and waste products. Because of this, the kidney is an inherently dynamic system where liquid flows are an important part of the organ’s function. In the study, the researchers integrated the cell-based model into a microphysiological system where the flow dynamics inside the kidney proximal tubule can be emulated. The researchers then used the combined system to test the reference compounds under both static and in vivo-like dynamic conditions.
“When we evaluated our model using reference compounds, we found that metallothioneins were reliably upregulated in the perfused cultures but not in static controls. The upregulation under dynamic conditions mirrors what is observed in vivo, demonstrating the validity of our model” says Eva-Maria.
The study is part of the project BIRDIE, which aims at creating three-dimensional in vitro models of the human renal tubulointerstitium and the results provide important benchmark data for future, more complex, tests using the dual-perfused platform. Furthermore, the new model will be integrated into TissUse’s product portfolio and offered to customers.
“The presented model is robust, easy to use, compatible with personalized medicine, and versatile, as it can also be used in multi-organ cultures. This high degree of flexibility of a perfused kidney model combined with a robust assay performance is new to the field” Eva-Maria concludes.
More about the research:
- The article A perfused iPSC-derived proximal tubule model for predicting drug-induced kidney injury is published in the journal Toxicology in vitro with Eva-Maria Dehne of TissUse GmbH as corresponding author.
- The research is part of the project Bioprinting on-chip microphysiological models of humanized kidney tubulointerstitium (BIRDIE), which is supported by the European Union’s Horizon 2020 FET Open programme under grant agreement No 964452.